 By Alan Pryor
By Alan Pryor
Public television viewers may have caught last week’s two-hour show about hexavalent chromium (chromium-6) in California drinking water. In part, the show discussed the continued efforts of the California Department of Public Health (CDPH) to impose a Maximum Contaminant Limit (MCL) on chromium-6 in drinking water throughout California…
Chromium is a naturally occurring metal in soils and natural waters. It exists in a variety of forms with chromium-6 being the most toxic to humans and wildlife. In addition to being a proven human carcinogen, when ingested chromium-6 exhibits a variety of toxic effects on animals including extensive damage to the gastrointestinal tract, liver, and kidneys.
Chromium-6 was the culprit chemical leaked by PG&E into the aquifer beneath Hinkley, California in the case made famous by Erin Brokovitch in the movie of the same name in the 1990s. However, its presence in that particular aquifer was due to man-made chromium and it was present in much higher concentrations than when naturally occurring such as in Davis well water,
The California Department of Public Health (CDPH) included chromium-6 as an unregulated chemical requiring monitoring in 2001. Based on recent data, 3,107 of 6,565 tested public wells have concentrations above the detection limit for purposes of reporting of 1 μg/L (micrograms per liter = parts per billion = ppb). Most detections of chromium-6 have occurred in Los Angeles, San Bernardino and Fresno Counties although it is also widely present in Yolo Co well waters.
Chromium-6 Levels in Davis Well Water
All of Davis’ wells have relatively stable concentrations of chromium-6 above this minimum detection level as measured over the past decade and shown in the following table. The deep aquifer wells have average concentrations from 1 to 16 μg/L while the intermediate aquifer wells have average concentrations ranging from 7 to 36 μg/L.
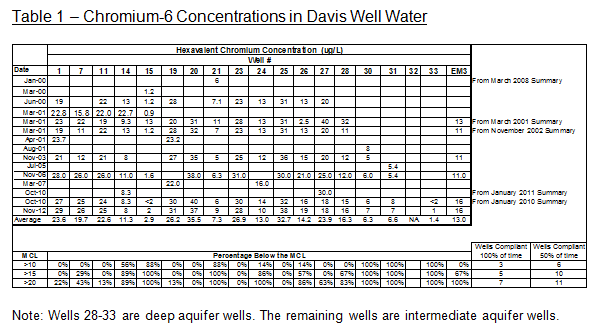
Note: Wells 28-33 are deep aquifer wells. The remaining wells are intermediate aquifer wells.
Dire Implications for Davis if an MCL is Promulgated for Chromium-6
As further discussed below, the best estimates of the new MCL that is expected to be ultimately set by CDPH for chromium 6 is between 10 and 20 μg/L. Any MCL set at these levels will have hugely dire consequences in terms of the City’s ability to continue to economically use the intermediate aquifer to provide potable water to citizens.
As shown in the bottom of Table 1 above, if the CDPH issues an MCL for chromium-6 at 20 μg/L or lower, the majority of Davis intermediate aquifer wells would be out-of-compliance with drinking water standards a majority of the time and would require remediation for the water to continue to be used for potable water purposes. If the standard were instead set at 10 ug/L, all but one of the intermediate aquifer wells and one deep aquifer well would be out-of compliance and require remediation for continued use.
Because of the minute quantities of chromium 6-in the water, the most widely available technology for removal of dissolved metals and minerals, reverse osmosis, could not be practically employed to remove this contaminant. The most viable alternative is a biological remediation process.
According to the most recent information available from the City’s Public Works department, each system of this type for an average-size intermediate aquifer well would cost between $4.5 million and $5 million per site! This is far in excess of the value of each well itself. Remediating only 10 of the 16 of the City’s 16 intermediate aquifer wells alone would thus cost from $45 million to $50 million!
As a result of the exorbitant cost of remediation, if the newly mandated MCL is promulgated as expected within several years, it would render the intermediate aquifer completely economically usable by the City for potable water purposes.
Opponents of Measure I who claim that continued pumping of the intermediate aquifer can be successfully managed to meet both our drinking water and waste water discharge requirements should rethink the factual basis of that claim in light of the stated objectives of the CDPH to meet their legislated mandates.
Because of the high levels of chromium in at least one deep aquifer well, it also calls into question the wisdom of future sole reliance on the deep aquifer for providing Davis drinking water. By contrast, all of the sample measurements reported for chromium-6 in the Sacramento River were below the threshold of detection (1 ug/L) according to the Clean Water Agency report entitled “Sacramento River Water Quality Assessment for the Woodland Davis Water Supply Project” , March, 2011.
Regulatory Process for Setting Chromium-6 MCL
Chromium-6 is currently regulated only under the 50 µg/l MCL standard for total chromium. California’s MCL for total chromium was established in 1977 to address exposures to chromium-6.
Subsequently, concerns about chromium-6’s potential carcinogenicity when ingested resulted in a state law that requires CDPH to adopt a chromium-6-specific MCL. California’s Health and Safety Code now requires CDPH to establish an MCL at a level as close as is technically and economically feasible to the contaminant’s Public Health Goal (PHG).
PHGs are defined as contaminant concentrations in drinking water that do not pose any significant risk to health and are developed by Cal/EPA. On July 27, 2011, CDPH established its PHG for chromium-6 at a concentration of 0.02 µg/L. The PHG represents a de minimis lifetime cancer risk from exposure to chromium-6 in drinking water based on studies in laboratory animals.
The availability of a final PHG enables CDPH to proceed with setting a primary drinking water standard (the MCL). Based on past experience, CDPH estimates the MCL development process will take approximately 18-24 months and is working to have an MCL developed and available for public comment by July 2013. The rulemaking process may then take an additional 12-24 months.
Assuming the process moves along without any major delays, an enforceable MCL would thus be established between July 2014 and July 2015. Although the actual MCL proposed by the CDPH will not be known for certainty until this coming July when the initial draft of the rule will be issued, based on extensive public meetings and review to date, it is expected that the MCL will be set at a level of 20 μg/L with a distinct possibility that it will be set even lower to 10 or 15 μg/L.
Fear mongering, and a bald-faced attempt to influence the election.
“The good thing about science is that it’s true whether or not you believe in it.” ― Neil deGrasse Tyson
Mike – you sound a bit like one of those global warming deniers. Science can certainly be used to frighten people. But the only challenge to make is whether the facts justify the claims. Are you making a case that we DON’T have a problem with too high levels of Chromium-6 in our intermediate aquifer, or are you making the case that it does not matter (e.g., is not worthy of fear or concern)?
According to this report by the Environmental Working Group on [b]Chromium-6 in US Tap Water[/b], the level of Chromium-6 in Sacramento’s tap water exceeds the proposed public health goal.
[b]http://static.ewg.org/reports/2010/chrome6/chrome6_report_2.pdf[/b]
In this EPA report the water quality in the Sacramento and Feather Rivers has been identified by the State of California as impaired by copper, mercury, toxicity and more than 15 pesticides including diazinon chlorpyrifos and lindane.
[b]http://www.epa.gov/region9/water/watershed/measurew/feather-[b]sac/index.html[/b][/b]
Where I think we are going is people are no longer going to drink tap water, not because of taste, but because of risk. It’s fine for showers and washing dishes and laundry etc., where most of it goes wherever you put your gray water, but ingesting appreciable amounts is going to be a problem. So while we chase the ever elusive drinking water standard, it’s a moving target that’s getting harder to attain every day.
it’s really true science gives the power if u used in good way if we do any mistake then it’s may be bad effect on we. Actually i am talking about Hexavalent It’s really useful but it’s contain many harmful Disadvantage..
Before reading this tutorials i have read this site http://www.appliedspeciation.com/Hexavalent-Chromium-Analysis.html
about Hexavalent i hope you read and know the impotence of Hexavalent..;-)